Why Does Potassium Fluoride Have a High Melting Point
Explain in terms of bonding why KBrF4 has a high melting point3 ionic bonds between ions strong electrostatic attraction between K and BrF4- ions. This energy is provided in the high melting temperatureCaO has a higher melting point than LiCl due to the Ca 2 ion.
Solved Select Four Physical Properties Of Potassium Fluoride Chegg Com
This structure requires a lot amount of energy to increase the intermolecular space in order to convert into a liquid or even gas.
. Thats why metal poses a high melting and boiling point. Which Would Have A Higher Melting Point Sodium Chloride Or Aluminum Oxide. Both hydrogen fluoride and ammonia show hydrogen bonding which elevates the boiling points of these two compounds when compared to the rest of the pnictogen and halogen hydrides.
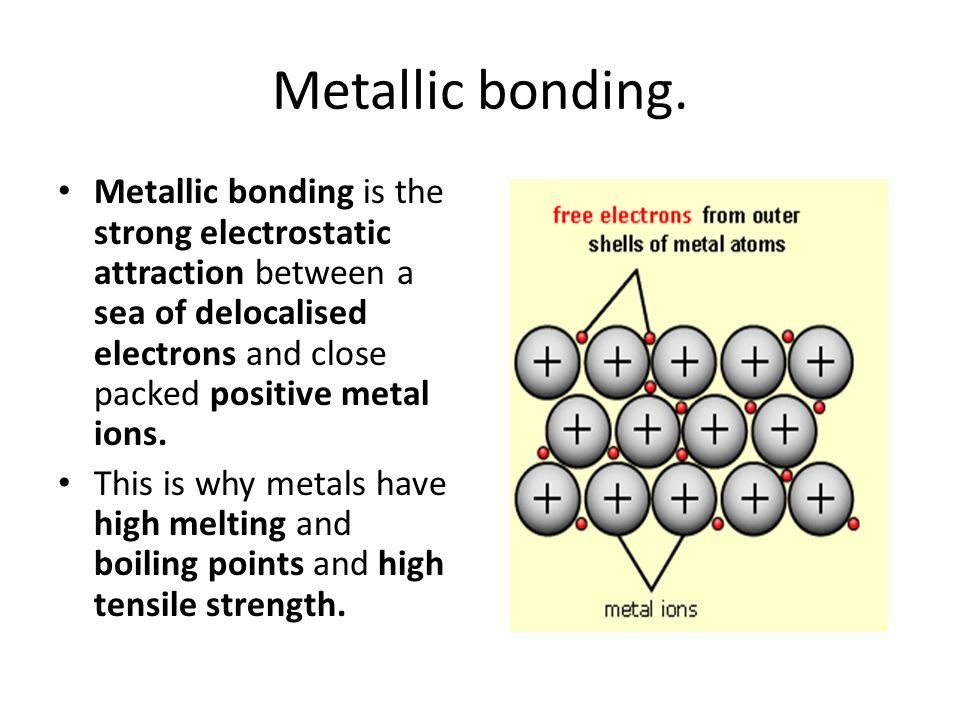
This is because the molecules are held together by strong molecular force of metallic bond to form a hard lattice structure. Since there will be more delocalized electrons per atom throughout calcium than potassium the bond strength is stronger in calcium than potassium. Since more energy is required to break the bonds the melting point of calcium will be higher than that of potassium.
Both are ionic solids. What Does High Melting Point Mean. The boiling points of fluorine and hydrogen fluoride are -188 C and 195 C.
Since the electrostatic forces of attraction between oppositely charged ions are strong their melting and boiling points are high. The material on this site can not be reproduced distributed transmitted cached or otherwise. How does this make any sense.
The strength of ionic bonds also depends on the distance between the ions bond length. Potassium fluoride KF or FK CID 522689 - structure chemical names physical and chemical properties classification patents literature biological activities. Why does magnesium fluoride has a higher melting point than fluorine and carbon tetrafluoride.
This ion has a greater charge than Li and so forms stronger attractions. Hence the high melting and boiling point. The reason for that comes from the.
Join Yahoo Answers and get 100 points today. What Mass Of Solute Is Contained In 417 ML Of A 0314 M Magnesium Fluoride Solution. Could someone point me in the right direction with these questions lads Alkali metal reactivity AQA Chemistry C2 15052014 Higher unofficial markscheme Science 6 mark question.
Cs Per Minute Chart Greys Anatomy Plane Crash Hot Ones Fiery Chipotle Mad Max 2021 Setting Houses Rent Dale City Va Marantz Imperial 7 Crossover Winium Automation Tool Categories. Why does MgO have a higher melting point than KCL. This requires more heat energy to overcome.
The difference in melting points for ionic compounds can be explained by the size of the ions. Since more energy is required to break the bonds the melting point of calcium will be. 815 G 196 G 260 G 623 G None Of These.
Does Is Mean Higher Chances Of Melting Or What. Because MgO Mg2 and O2 has ions with greater charges than NaF Na and F it takes more energy to break the bonds in MgO resulting in a higher melting point. To be specific it varies inversely with the square of the center-to-center distance between the ions.
Why does Sodium Fluoride NaF have a high melting pointboiling point. Potassium fluoride is held together by a strong ionic bond and typically molecules with strong ionic bonds have higher melting points. For example among the potassium halides the melting point is lowest for the iodide 681 oC and highest for the fluoride 858oC.
Why does MgO melt higher than NaF. What Is The Melting Point Of Salt. Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions.
The melting point of hydrogen fluoride is -836C as compared to that of ammonia which is -7773C. Why does calcium fluoride have a high melting point show 10 more. How Much Fluoride Is.
Since there will be more delocalized electrons per atom throughout calcium than potassium the bond strength is stronger in calcium than potassium. Explaining melting points It takes a lot of energy to overcome the strong electrostatic forces of attraction between oppositely charged ions so ionic compounds have high melting and. These attractions are strong and so require a large amount of energy to break.
BrF4- ions are also formed when potassium fluoride dissolves in liquid BrF3 to form KBrF4. Why does lithium chloride have a high melting point February 16 2021. So a larger energy is needed to separate the atoms from solid state to liquid state and hence KF has higher melting point than KCl.
Answer 1 of 2. All ionic compounds have high melting points for this reason. Potassium is somewhat less electronegative than sodium but its ion is substantially bi.
That means that there are sometimes predictable variations in the properties of ionic compounds.

Give Reasons For The Following Lii Has Lower Melting Point Than Lif
I Was Taught That Melting Point Increases As Ionic Bonds Strengthen And Ionic Bonds Strengthen As Electronegativity Difference Between Two Atoms Increases So Why Does Sodium Fluoride Have A Higher Melting Point

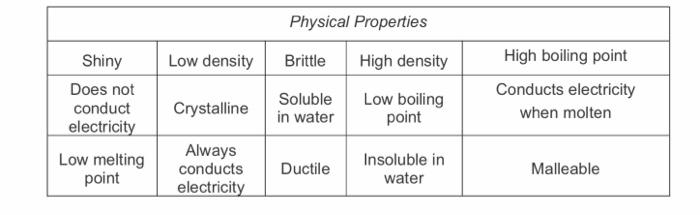
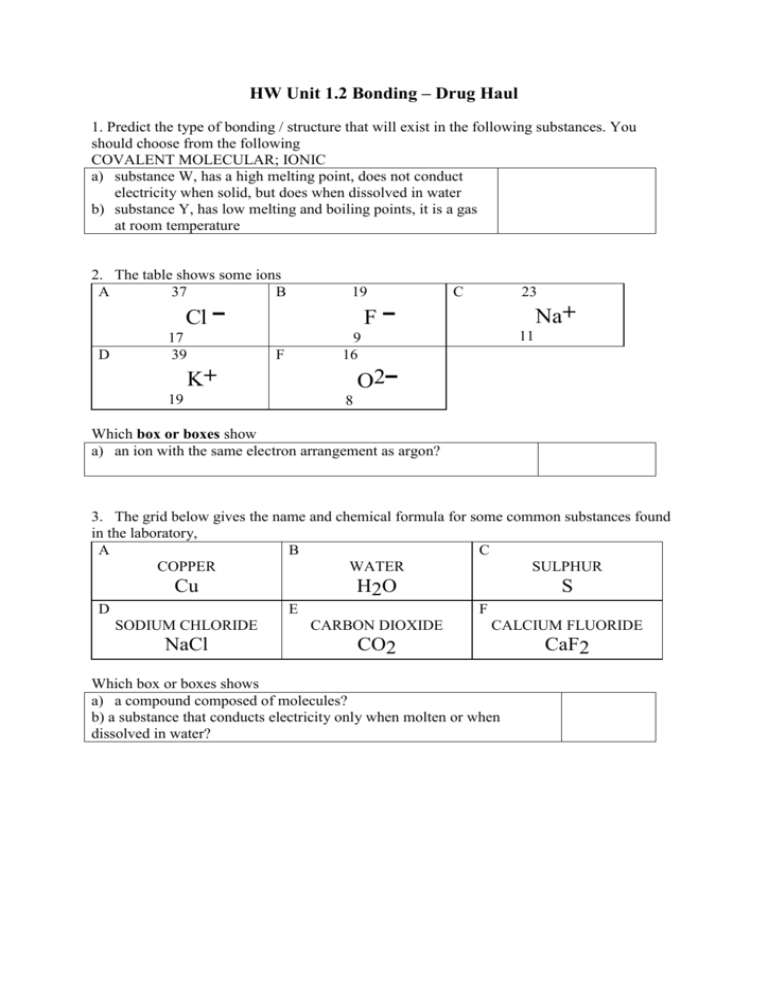
Drug Haul Bonding Revision Questions

Ionic Compounds Noadswood Science Ppt Video Online Download

No Comp Book Question Write Down Your Homework Ppt Download

Why Dies Potassium Fluoride Have A High Melting Point

Aqa Gcse 9 1 Chemistry For Combined Science Trilogy By Collins Issuu
I Was Taught That Melting Point Increases As Ionic Bonds Strengthen And Ionic Bonds Strengthen As Electronegativity Difference Between Two Atoms Increases So Why Does Sodium Fluoride Have A Higher Melting Point

Melting And Boiling Points Of Select Salt Compounds Download Table

Solved Explain For Each Observation With Appropriate Atomic Chegg Com
Why Does Fluorine Have A High And Low Melting And Boiling Point Yet Its Atoms Are Joined By Covalent Bonding Quora

Why Does Potassium Fluoride Have A Higher Melting Point


Comments
Post a Comment